Oxygen accounts for nearly half of the mass of the earth s crust two thirds of the mass of the human body and nine tenths of the mass of water.
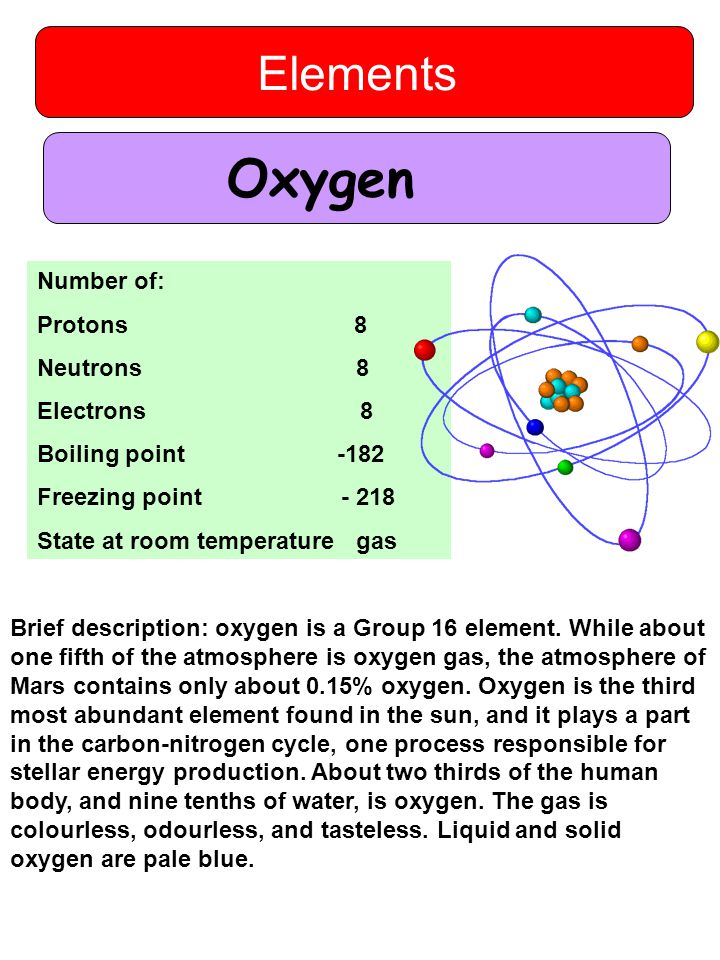
Oxygen element state at room temperature.
Its monatomic form h is the most abundant chemical substance in the universe constituting roughly 75 of all baryonic mass.
It is the rarest naturally occurring element in the earth s crust occurring only as the decay product of various heavier elements.
Other liquid elements.
It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compounds after hydrogen and helium oxygen is the third most abundant element in the universe by mass.
With a standard atomic weight of circa 1 008 hydrogen is the lightest element on the periodic table.
A sample of the pure element has never been assembled because any macroscopic.
The most stable is astatine 210 with a half life of 8 1 hours.
Oxygen is the third most abundant element in the universe and makes up nearly 21 of the earth s atmosphere.
Oxygen is vital for respiration which is the process that transfers energy from glucose to.
Several of the nonmetals are gases in their elemental form.
You re breathing it in right now with a large portion of nitrogen too.
That state of matter of an element may be predicted based on its phase diagram.
All of astatine s isotopes are short lived.
Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure the chemical symbol for hydrogen is h.
The element oxygen is a gas at room temperature.
When pressure is controlled other pure elements may be found at room temperature.
At standard temperature and pressure two.
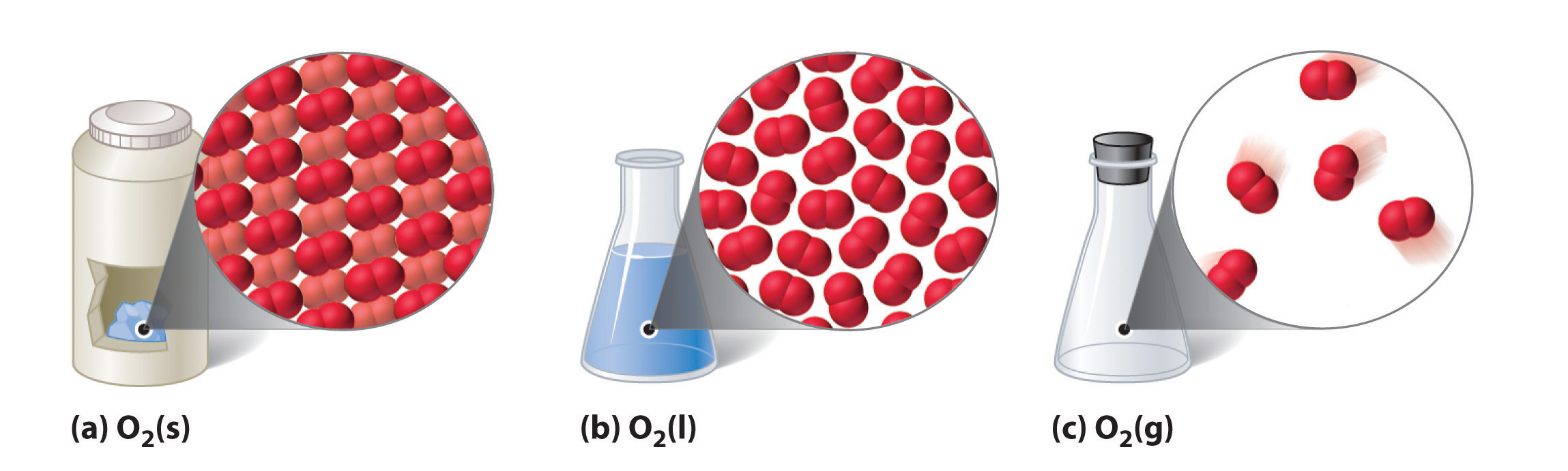
It is a liquid at 90 k and a solid at 54 k.
Gas oxygen o 2 is a gas at room temperature 273 k.
Oxygen is a non metal element that is a gas at room temperature.
The temperature at which the liquid gas phase change occurs.
An example is the halogen element chlorine.
While temperature is an easily controlled factor manipulating pressure is another way to cause a phase change.
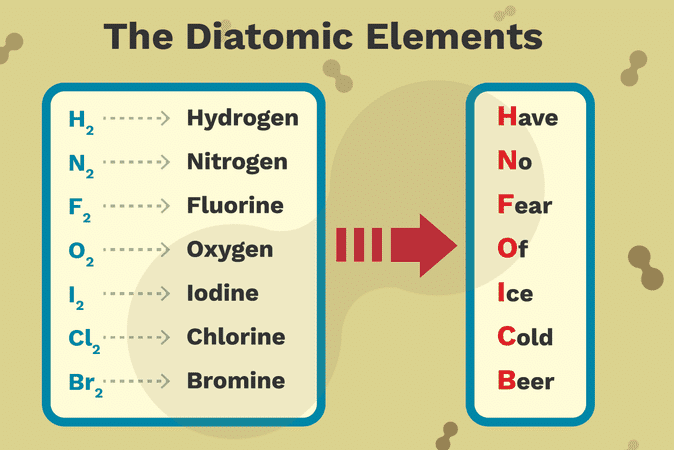
Elemental hydrogen h element 1 nitrogen n element 7 oxygen o element 8 fluorine f element 9 and chlorine cl element 17 are all gases at room temperature and are found as diatomic molecules h 2 n 2 o 2 f 2 cl 2.
Relative atomic mass the mass of an atom relative to that of.
Its molecules contain two oxygen atoms.